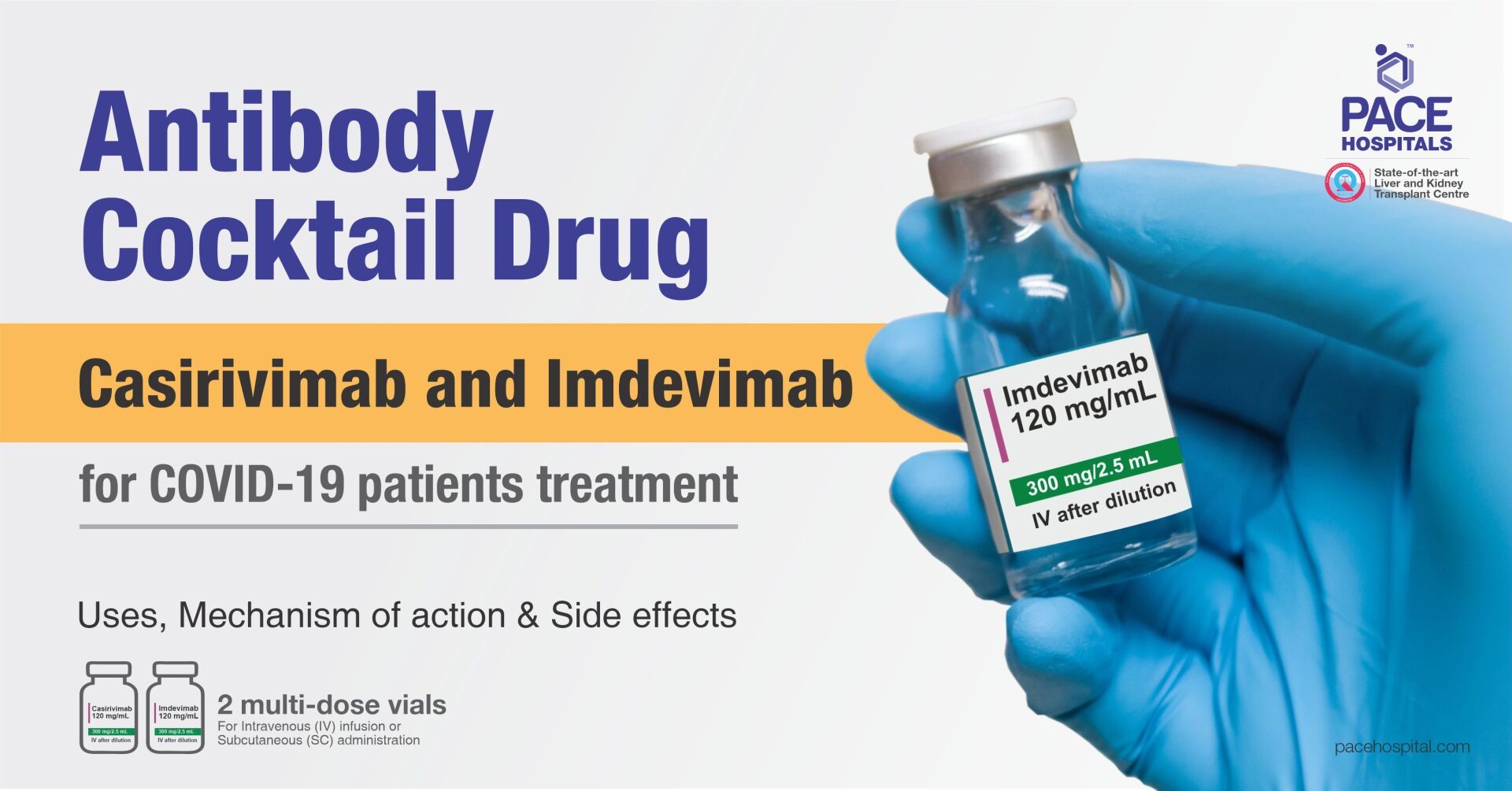
Casirivimab and Imdevimab (Antibody Cocktail Drug) - Uses, Side effects and Cost
Antibody Cocktail Drug - Casirivimab and Imdevimab is specified for restricted use in emergency conditions to treat COVID-19 patients having mild to moderate symptoms and who are at high risk of developing severe symptoms of COVID-19 (Coronavirus). Recently the Central Drugs Standards Control Organisation (CDSCO) had provided a EUA (emergency use authorization) for antibody cocktail drug in India, previously it also received emergency use authorization in the US and other European countries.
Roche India and Cipla Limited announced on 24 March 2021 that Antibody Cocktail (Casirivimab and Imdevimab) will be available in India for patients with mild and moderate symptoms of COVID-19. Cipla limited will be distributing the drug through its strong distribution channel across the country, also mentioned drug will be available through leading hospitals those who are providing treatment to COVID patients. Emergency use authorization of Casirivimab and Imdevimab in India can help in reducing hospitalization of COVID-19 patients those who are at high risk of developing severe symptoms.
What is Casirivimab and Imdevimab (Antibody Cocktail Drug) and its mechanism of action?
Casirivimab and Imdevimab are human immunoglobulin G-1 (IgG1) monoclonal antibodies that are laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens such as viruses. These are specifically directed against the spike protein S1 and S2 of SARS-CoV-2, designed to block the virus’ attachment and entry into human cells.
Monoclonal antibodies therapies may help high risk COVID-19 patients to avoid hospitalization. Casirivimab and Imdevimab cocktail remains efficacious against the widest spread variants and reduces the risk of losing its neutralization potency against new emerging variants.
Casirivimab and Imdevimab is mandatory and must be done by the prescribing healthcare provider should be administered by a qualified healthcare professional. Casirivimab and Imdevimab must be administered together after dilution by intravenous (IV) infusion. The intravenous (IV) administration takes about 20 to 30 minutes.
Casirivimab and Imdevimab uses -
Antibody Cocktail Drug (Casirivimab and Imdevimab) is to be administered together to treat COVID-19 patients having mild to moderate symptoms in adults and pediatric patients 12 years of age and older, weighing at least 40 kg who are tested SARS-CoV-2 positive, and who are at high risk of developing severity of COVID-19 and / or hospitalization simultaneously don’t require oxygen. Phase 3 trial of Casirivimab and Imdevimab shown better results on high risk COVID-19 patients, reduced the risk of hospitalization and mortality by 70%, and shortened the coronavirus symptoms by 4 days.
The safety and effectiveness of this monoclonal antibody therapy for use in the treatment of COVID-19 continues to be evaluated. The authorized dosage is 1,200 mg as combined dose where 600 mg of casirivimab and 600 mg of imdevimab administered together by intravenous (IV) infusion or subcutaneous route as soon as possible after positive viral test for SARS-CoV-2 and within 10 days of symptoms onset.
Casirivimab and Imdevimab are not authorized for use in patients those who comes these categories:
- Require oxygen therapy due to COVID-19
- Hospitalized due to COVID-19
- Require an increase in baseline oxygen flow rate due to COVID-19 in those on chronic oxygen therapy due to underlying non-COVID-19 related comorbidity
Patients treated with Casirivimab and Imdevimab should continue to self-isolate and use precautions (wear mask, self-isolation, social distance, frequent handwashing and maintain hygiene) and preventions to stop further spread.
Who all are at high risk in COVID-19 and should get Casirivimab and imdevimab monoclonal antibodies treatment?
COVID-19 patients are at high risk if having at least one of the following conditions-
- Older age group >=60 years
- Age group >=55 years and having hypertension or cardiovascular disease or chronic obstructive pulmonary disease / other chronic respiratory disease.
- Age between 12 and 17 years and having BMI ≥85th percentile or congenital or acquired heart disease or sickle cell disease or neurodevelopmental disorders such as cerebral palsy or gastrostomy or tracheostomy or asthma, reactive airway or other chronic respiratory disease that requires daily medication for control.
- Type 1 or type 2 diabetes mellitus
- Obese and having body mass index (BMI) ≥35
- Chronic kidney disease
- Immunosuppressive disease such as cancer, sickle cell anemia, bone marrow or organ transplantation, thalassemia, HIV or immune deficiencies
- Currently on immunosuppressive treatment
- Hypertension
- Asthma
- Cardiovascular disease
- Chronic lung disease
- Chronic liver disease
What is the price / cost of Casirivimab and Imdevimab in India?
The price for each patient dose is INR 59,750 inclusive of all taxes. Each dose consists 600 mg of Casirivimab and 600 mg of Imdevimab; 1200 mg as a combined dose.
Each pack of Antibody Cocktail (Casirivimab and Imdevimab) contains one vial of Casirivimab (1200 mg) and one vial of Imdevimab (1200 mg), can treat two patients as a combined dose of 1200 mg per patient. The maximum retail price for each pack is INR 1,19,500 inclusive of all taxes.
Where to get antibody cocktail injection in Hyderabad (Casirivimab and Imdevimab)?
We Pace Hospitals have started administering antibody cocktail injection for COVID patients at our Hitech City, Branch located near Hitech City Metro Station, Hyderabad, Telangana, India. Locate Us
What are the side effects of Antibody cocktail drug (Casirivimab and Imdevimab)?
There are limited clinical data available for casirivimab and imdevimab. Serious and unexpected adverse events may occur that have not been previously reported with casirivimab and imdevimab use.
Request an appointment
Fill in the appointment form or call us instantly to book a confirmed appointment with our super specialist at 04048486868
Appointment request - health articles
Thank you for contacting us. We will get back to you as soon as possible. Kindly save these contact details in your contacts to receive calls and messages:-
Appointment Desk: 04048486868
Whatsapp: 8977889778
Regards,
Pace Hospitals
Hitech City and Madinaguda
Hyderabad, Telangana, India.
Oops, there was an error sending your message. Please try again later. We will get back to you as soon as possible. Kindly save these contact details in your contacts to receive calls and messages:-
Appointment Desk: 04048486868
Whatsapp: 8977889778
Regards,
Pace Hospitals
Hitech City and Madinaguda
Hyderabad, Telangana, India.
Our Locations – Find the Best Hospital Near You
Metro Pillar Number C1772, Beside Avasa Hotel, Hitech City Road, Near HITEC City Metro Station, Hyderabad, Telangana, India.
Mythri Nagar, Beside South India Shopping Mall, Hafeezpet, Madeenaguda, Hyderabad, Telangana, India.
040 4848 6868
Payment in advance for treatment at PACE Hospitals, Hyderabad, Telangana, India (Pay in INR ₹)
For Bank Transfer:-
- Bank Name: HDFC
Company Name: Pace Hospitals
A/c No.50200028705218
IFSC Code: HDFC0000545 - Bank Name: STATE BANK OF INDIA
Company Name: Pace Hospitals
A/c No.62206858997
IFSC Code: SBIN0020299
Scan QR Code by Any Payment App (GPay, Paytm, Phonepe, BHIM, Bank Apps, Amazon, Airtel, Truecaller, Idea, Whatsapp etc).

CONTACT US
Call: +914048486868
WhatsApp: +918977889778
Email: info@pacehospitals.in
FOLLOW US
SUBSCRIBE
Subscribe to our newsletter and stay updated with the latest health information.
Subscribe to PACE Hospitals' Public Newsletter
Thank you for subscribing to PACE Hospitals' Newsletter. Stay updated with the latest health information.
Oops, there was an error. Please try again submitting your details.
ABOUT US
QUICK LINKS
Disclaimer
General information on healthcare issues is made available by PACE Hospitals through this website (www.pacehospital.com), as well as its other websites and branded social media pages. The text, videos, illustrations, photographs, quoted information, and other materials found on these websites (here by collectively referred to as "Content") are offered for informational purposes only and is neither exhaustive nor complete. Prior to forming a decision in regard to your health, consult your doctor or any another healthcare professional. PACE Hospitals does not have an obligation to update or modify the "Content" or to explain or resolve any inconsistencies therein.
The "Content" from the website of PACE Hospitals or from its branded social media pages might include any adult explicit "Content" which is deemed exclusively medical or health-related and not otherwise. Publishing material or making references to specific sources, such as to any particular therapies, goods, drugs, practises, doctors, nurses, other healthcare professionals, diagnoses or procedures is done purely for informational purposes and does not reflect any endorsement by PACE Hospitals – your trusted hospital near me.